Freshers Jobs Production QA QC Engineering EHS at Ipca Laboratories February 2026 Madhya Pradesh.
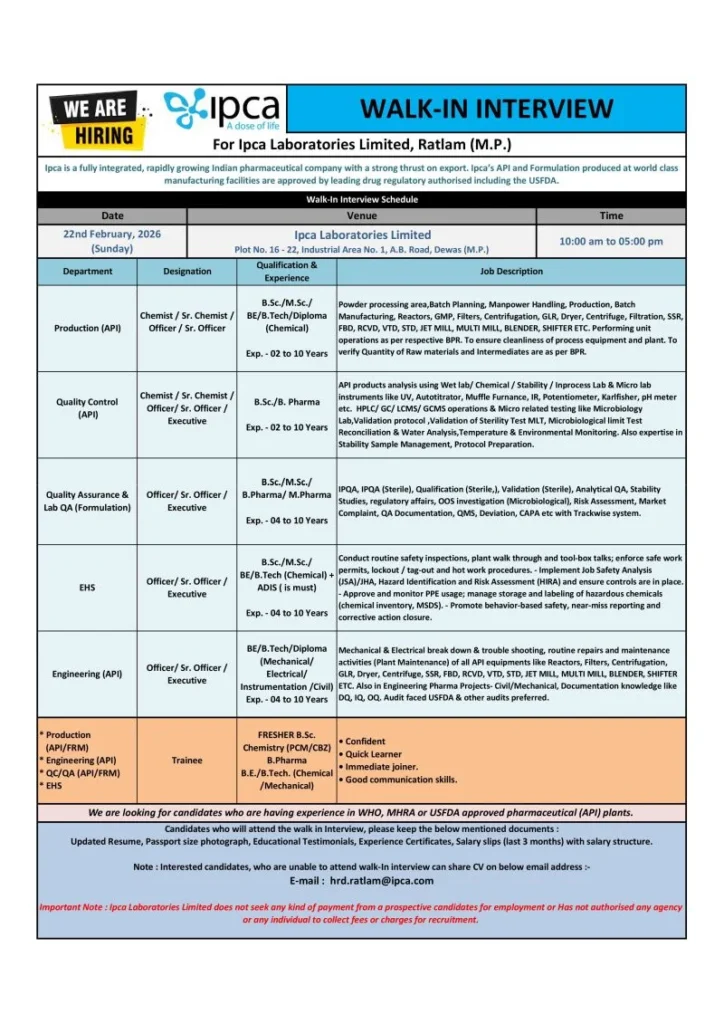
Ipca Laboratories Limited has announced a large-scale walk-in recruitment drive for multiple departments across its pharmaceutical manufacturing operations. The company is inviting both freshers and experienced professionals for roles in Production, Quality Control, Quality Assurance, Engineering, and EHS functions. This hiring initiative offers an excellent opportunity for candidates seeking careers in regulated pharmaceutical environments.
The walk-in interview scheduled for February 2026 is especially beneficial for those aiming to work in API manufacturing and compliance-driven pharmaceutical facilities. Candidates with relevant educational backgrounds in pharmacy, chemistry, or engineering can explore multiple roles across technical and operational domains. Freshers Jobs Production QA QC Engineering EHS
Company Overview
Ipca Laboratories Limited is a globally recognized pharmaceutical company with a strong presence in APIs and finished formulations. The company operates multiple manufacturing facilities approved by international regulatory agencies, including USFDA and other global authorities. With decades of experience in pharmaceutical manufacturing, Ipca offers structured career growth opportunities across production, quality, and engineering functions.
Working with a regulated pharma manufacturer allows professionals to gain exposure to global quality standards, audit-driven systems, and large-scale manufacturing practices. Freshers Jobs Production QA QC Engineering EHS
Job Location & Employment Type
- Primary Work Location: Ratlam, Madhya Pradesh
- Interview Location: Dewas, Madhya Pradesh
- Employment Type: Full-time roles and trainee opportunities
- Hiring Mode: Walk-in Interview
This recruitment drive includes openings for both freshers and experienced professionals across multiple pharmaceutical departments. Freshers Jobs Production QA QC Engineering EHS
Open Positions / Department Details
Production (API)
- Roles: Chemist, Senior Chemist, Officer
- Experience Range: 2–10 years
- Educational Background: Chemical sciences and engineering-related qualifications
Quality Control (API)
- Roles: Chemist / Executive
- Experience Range: 2–10 years
- Focus: Analytical testing and instrument-based evaluation
Quality Assurance (Formulation & Lab QA)
- Roles across IPQA and quality systems
- Experience Range: 4–10 years
- Focus: QMS, compliance, and regulatory documentation
Engineering (API Plant)
- Roles in maintenance and plant engineering functions
- Disciplines: Mechanical, Electrical, Instrumentation, Civil
EHS (Environment, Health & Safety)
- Role: EHS Officer
- Specialized requirement: Safety certification background
Trainee Roles (Freshers)
- Entry-level positions for candidates starting pharma careers
- Designed for graduates seeking practical exposure in pharma manufacturing
Key Roles & Responsibilities
Production (API Manufacturing)
- Managing batch manufacturing and powder processing operations
- Handling reactors, dryers, centrifuges, and filtration equipment
- Supporting manpower planning and production scheduling
- Ensuring adherence to GMP during manufacturing activities
- Maintaining equipment cleaning records and batch documentation
Quality Control Responsibilities
- Conducting API testing using analytical instruments
- Operating equipment such as HPLC, GC, UV, and related tools
- Supporting stability testing and sample management
- Performing environmental and water analysis where required
- Assisting in validation and routine analytical documentation
Quality Assurance Responsibilities
- Performing IPQA activities in sterile and non-sterile areas
- Managing deviation handling and CAPA documentation
- Supporting risk assessment and quality management systems
- Investigating OOS and maintaining regulatory documentation
- Supporting stability and compliance programs
Engineering Responsibilities
- Handling preventive and breakdown maintenance activities
- Maintaining reactors and process equipment
- Supporting qualification documentation such as DQ, IQ, and OQ
- Troubleshooting plant engineering issues
- Supporting regulatory audit readiness where applicable
EHS Responsibilities
- Conducting plant safety audits and inspections
- Implementing hazard identification and risk assessment systems
- Managing safety permits and compliance protocols
- Ensuring PPE adherence across plant areas
- Promoting safety awareness and best practices
Freshers Jobs Production QA QC Engineering EHS
Eligibility Criteria
Education
Candidates must hold relevant qualifications depending on department:
- B.Sc / M.Sc (Chemistry or related fields)
- B.Pharm / M.Pharm
- Diploma in Engineering
- BE / B.Tech (Chemical, Mechanical, Electrical, Instrumentation, Civil)
For EHS roles, safety certifications such as ADIS may be required.
Experience
- Freshers eligible for trainee roles
- 2–10 years of experience for technical roles in production or QC
- 4–10 years preferred for QA, Engineering, and EHS positions
- Experience in regulated pharmaceutical plants is advantageous
Skills Required
- Understanding of GMP and regulatory expectations
- Technical knowledge of pharmaceutical manufacturing or quality systems
- Analytical instrument handling (for QC roles)
- Documentation and compliance awareness
- Strong safety and process discipline
- Team collaboration and operational flexibility
Candidates with exposure to regulated markets such as USFDA-approved plants may have an added advantage. Freshers Jobs Production QA QC Engineering EHS
Salary & Benefits
- Estimated salary range: ₹2.5 LPA to ₹9.5 LPA depending on role and experience
- Opportunity to work in a regulated pharmaceutical environment
- Exposure to global audit and compliance standards
- Career growth in API manufacturing and quality domains
- Learning opportunities in validation and regulatory systems
Actual compensation will vary based on experience, skill set, and role fitment.
Selection Process
The recruitment process will be conducted through an on-the-spot walk-in interview, which may include: Freshers Jobs Production QA QC Engineering EHS
- Resume screening at the venue
- Technical discussion with departmental panel
- Final HR interaction
Candidates with relevant experience and clarity in technical fundamentals may receive quicker selection outcomes.
Walk-In Interview Details
- Date: 22nd February 2026 (Sunday)
- Time: 10:00 AM to 05:00 PM
- Interview Venue: Plot No. 16–22, Industrial Area No. 1, A.B. Road, Dewas, Madhya Pradesh
- Work Location: Ratlam (M.P.)
Applicants should reach early with updated resumes and supporting documents to avoid last-minute delays. Freshers Jobs Production QA QC Engineering EHS
How to Apply
Candidates interested in this opportunity can directly attend the walk-in interview at the specified venue. Freshers Jobs Production QA QC Engineering EHS
Documents to carry:
- Updated resume
- Educational certificates
- Experience proof (if applicable)
- ID proof and photographs
Early arrival is recommended for smoother participation in the recruitment process. Freshers Jobs Production QA QC Engineering EHS
Important Dates
- Walk-In Interview Date: 22 February 2026
- Mode of Application: Direct walk-in
- Hiring Coverage: Freshers and experienced professionals
Candidates are advised to prepare documents in advance and plan travel accordingly. Freshers Jobs Production QA QC Engineering EHS
Why Apply for This Pharma Job?
Ipca Laboratories offers a strong platform for professionals looking to build careers in regulated pharmaceutical manufacturing. This recruitment drive is particularly valuable because it includes both entry-level and experienced roles across multiple departments, allowing candidates from diverse backgrounds to participate. Freshers Jobs Production QA QC Engineering EHS
Working in API manufacturing environments provides exposure to high-quality production systems, global regulatory standards, and advanced manufacturing equipment. For freshers, trainee roles offer an excellent foundation for long-term growth in the pharmaceutical industry. For experienced professionals, this walk-in presents opportunities to transition into a globally recognized pharma organization with structured quality systems.
Such large-scale hiring drives also provide faster hiring outcomes compared to traditional application-based recruitment, making them ideal for candidates seeking immediate career opportunities.
Important Note
IndiaPharmaJobs.in is an independent job information platform that publishes verified pharmaceutical career updates for job seekers. We are not associated with Ipca Laboratories Limited or involved in the hiring process. Applicants should attend the walk-in interview directly and verify all details independently before applying. Freshers Jobs Production QA QC Engineering EHS
Final Words
If you are planning to build or advance your career in pharmaceutical manufacturing, this walk-in drive by Ipca Laboratories presents a valuable opportunity. With openings across Production, QA, QC, Engineering, and EHS departments, both freshers and experienced professionals can explore suitable roles. Candidates who meet the eligibility criteria should prepare their documents and attend the walk-in interview on the scheduled date to maximize their chances of selection. Freshers Jobs Production QA QC Engineering EHS


