Quality Assurance Jobs at Micro Labs in Bangalore
Company Overview
Micro Labs Limited is one of India’s most established pharmaceutical companies with a strong reputation for quality, compliance, and patient-centric manufacturing. The organization operates multiple WHO-GMP and globally regulated manufacturing facilities and supplies pharmaceutical products across domestic and international markets. Micro Labs is widely recognized for its robust quality systems, regulatory readiness, and continuous improvement culture. Quality Assurance Jobs at Micro Labs
The sterile manufacturing facility located at Bommasandra, Bangalore, is a critical unit supporting injectable and sterile dosage forms. Professionals working at this site gain extensive exposure to global regulatory audits, advanced quality systems, and sterile manufacturing compliance, making it an ideal workplace for experienced Quality Assurance professionals seeking long-term career stability and growth. Quality Assurance Jobs at Micro Labs
Job Location & Employment Type
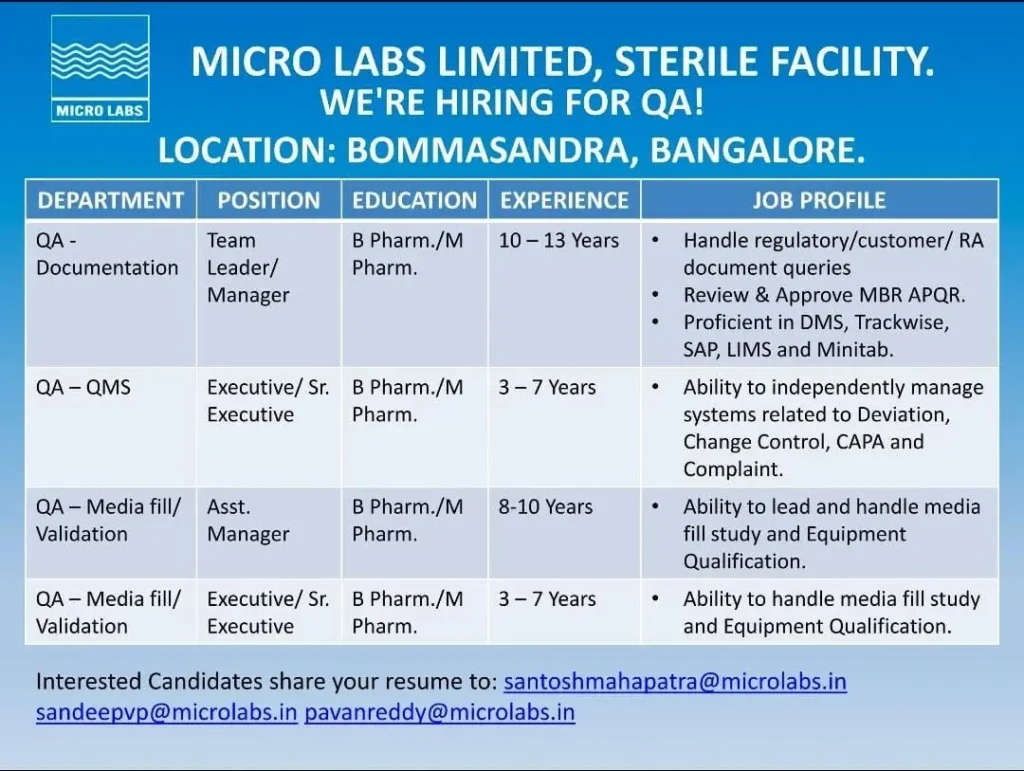
- Job Location: Bommasandra, Bangalore, Karnataka
- Employment Type: Full-time, permanent positions
- Industry Segment: Sterile / Injectable Pharmaceutical Manufacturing
Open Positions / Department Details
Micro Labs Limited is hiring experienced professionals across multiple roles in the Quality Assurance (QA) Department, specifically focusing on Documentation, QMS, Media Fill, and Validation functions. Quality Assurance Jobs at Micro Labs
QA – Documentation / QA-QMS
Team Leader / Manager
- Experience: 10 to 13 years
Executive / Senior Executive
- Experience: 3 to 7 years
QA – Media Fill / Validation
Assistant Manager
- Experience: 8 to 10 years
Executive / Senior Executive
- Experience: 3 to 7 years
These roles are based at the sterile manufacturing facility and require prior experience in injectable or sterile pharmaceutical environments.
Key Roles & Responsibilities
QA – Documentation / QA-QMS
Team Leader / Manager Responsibilities:
- Managing regulatory, customer, and internal documentation requirements
- Reviewing and approving MBRs, APQRs, SOPs, and quality system documents
- Overseeing quality management systems and continuous improvement initiatives
- Driving compliance through effective implementation of QMS procedures
- Supporting internal audits, customer audits, and regulatory inspections
- Ensuring effective utilization of electronic quality systems
Executive / Senior Executive Responsibilities:
- Handling deviations, change controls, CAPA, and customer complaints
- Conducting investigations and ensuring timely closure of quality events
- Reviewing documentation to ensure GMP and regulatory compliance
- Coordinating with cross-functional teams to maintain quality standards
- Supporting audit preparation and compliance monitoring activities
Quality Assurance Jobs at Micro Labs
QA – Media Fill / Validation
Assistant Manager Responsibilities:
- Planning, execution, and evaluation of media fill simulations
- Leading validation and qualification activities for sterile processes
- Ensuring sterility assurance and regulatory compliance
- Reviewing validation protocols, reports, and risk assessments
- Supporting regulatory inspections and audit responses
Executive / Senior Executive Responsibilities:
- Executing media fill studies under aseptic conditions
- Performing equipment qualification and requalification activities
- Preparing and reviewing validation documentation
- Coordinating with production, engineering, and QA teams
- Maintaining compliance with sterile manufacturing guidelines
Quality Assurance Jobs at Micro Labs
Eligibility Criteria
Education
Candidates must hold one of the following qualifications from a recognized institution:
- B.Pharm
- M.Pharm
Relevant specializations may include Quality Assurance, Pharmaceutics, Industrial Pharmacy, Pharmaceutical Technology, Regulatory Affairs, Validation Sciences, or related pharmaceutical disciplines.
Experience
- Executive / Senior Executive: 3 to 7 years
- Assistant Manager: 8 to 10 years
- Team Leader / Manager: 10 to 13 years
Skills Required
- Hands-on experience in sterile or injectable pharmaceutical manufacturing
- Strong understanding of GMP, QMS, and regulatory requirements
- Practical exposure to media fill, validation, and documentation systems
- Working knowledge of electronic systems such as DMS, TrackWise, SAP, LIMS, and Minitab
- Audit readiness and inspection handling experience
- Strong documentation, investigation, and compliance skills
Quality Assurance Jobs at Micro Labs
Salary & Benefits
- Salary: As per industry standards and company norms
- Competitive compensation based on experience and role level
- Opportunity to work in an advanced sterile manufacturing facility
- Exposure to global regulatory audits and quality systems
- Long-term career growth and professional stability
Quality Assurance Jobs at Micro Labs
Selection Process
The recruitment process may include:
- Resume shortlisting based on experience and role suitability
- Technical interview with QA leadership
- HR discussion and final selection
- Appointment subject to verification and company policies
How to Apply
Eligible candidates can apply by sharing their updated resumes via email application only to the official email IDs mentioned below:
Applicants are advised to clearly mention the applied QA role and experience level in the email subject line. Shortlisted candidates will be contacted for further interview rounds.
Important Dates
- Last Date to Apply: Not specified (apply at the earliest)
Why Apply for This Pharma Job?
Quality Assurance roles at Micro Labs Limited offer experienced professionals the opportunity to work in a highly regulated sterile manufacturing environment with strong exposure to global quality systems. These positions are ideal for candidates seeking long-term career growth, regulatory expertise, and leadership opportunities in QA documentation, QMS, and validation. Being part of Micro Labs means contributing directly to patient safety, product quality, and healthcare advancement. Quality Assurance Jobs at Micro Labs
Important Note / Disclaimer
IndiaPharmaJobs.in is an independent pharmaceutical job information platform and is not affiliated with Micro Labs Limited or any other organization. All job details are shared for informational purposes only. Candidates are advised to verify recruitment information directly with the employer before applying. Quality Assurance Jobs at Micro Labs
Final Call-to-Action
If you are an experienced QA professional with sterile manufacturing exposure and are looking for a stable, growth-oriented role in Bangalore, this opportunity at Micro Labs Limited is an excellent career move. Eligible candidates are encouraged to apply promptly and take the next step in their pharmaceutical quality assurance career. Quality Assurance Jobs at Micro Labs


