Freshers Jobs for Production QA QC Microbiology at Micro Labs in Bangalore sterile formulation facility for freshers and experienced.
Company Overview
Micro Labs Limited is a well-established Indian pharmaceutical manufacturer known for its strong presence in domestic and international markets. The company operates advanced manufacturing facilities compliant with global regulatory standards and specializes in formulations including sterile injectables and ophthalmic preparations. Freshers Jobs for Production QA QC Microbiology
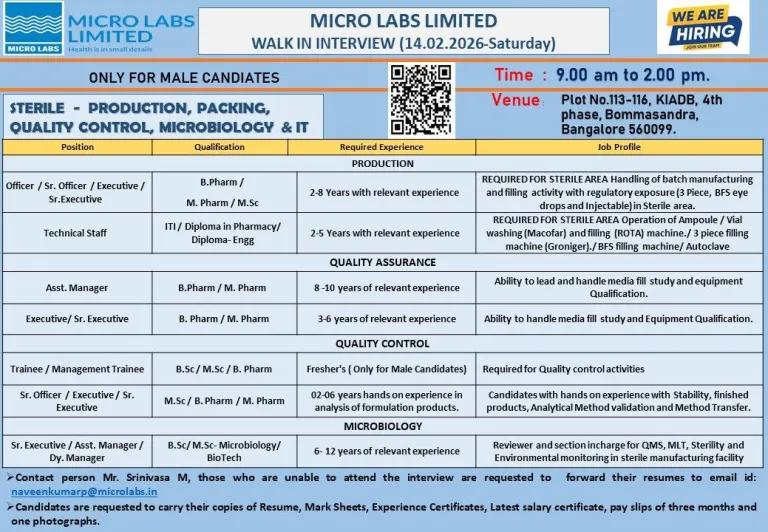
Micro Labs is conducting a major walk-in recruitment drive for its sterile formulation facility in Bangalore. This opportunity is suitable for both fresh graduates and experienced pharma professionals seeking growth in production, quality assurance, quality control, and microbiology departments.
Job Location & Employment Type
- Job Location: Bangalore, Karnataka
- Facility Area: Bommasandra Industrial Area
- Employment Type: Full-Time
- Industry: Pharmaceutical Manufacturing (Sterile Formulations)
Open Positions / Department Details
Multiple vacancies are available in the following departments:
Production – Sterile Formulation
Roles Available: Officer, Senior Officer, Executive, Senior Executive, Technical Staff
Quality Assurance (QA)
Roles Available: Assistant Manager, Executive, Senior Executive
Quality Control (QC)
Roles Available: Trainee, Management Trainee, Senior Officer, Executive
Microbiology
Roles Available: Senior Executive, Assistant Manager, Deputy Manager
The hiring drive focuses on sterile manufacturing operations including injectables, BFS ophthalmic preparations, and related production systems. Freshers Jobs for Production QA QC Microbiology
Key Roles & Responsibilities
Production (Sterile Area)
- Manage batch manufacturing and sterile filling activities
- Operate equipment such as vial washing machines, filling machines, BFS systems, autoclaves, and related sterile equipment
- Handle media fill operations and ensure compliance with sterile process validation
- Maintain batch records and ensure adherence to cGMP standards
Quality Assurance
- Support equipment qualification and validation activities in sterile areas
- Manage media fill studies and review qualification protocols
- Ensure documentation compliance and regulatory readiness
- Oversee QMS implementation in sterile manufacturing
Quality Control
- Perform testing of raw materials, in-process samples, and finished products
- Conduct stability studies and analytical method validation
- Support method transfer and documentation activities
- Ensure compliance with laboratory SOPs and regulatory standards
Microbiology
- Supervise microbiological testing such as sterility testing, MLT, and environmental monitoring
- Review microbiology reports and ensure QMS compliance
- Manage section activities and support audit readiness
- Oversee contamination control practices in sterile manufacturing
All roles require strict adherence to cGMP, regulatory documentation standards, and safety guidelines. Freshers Jobs for Production QA QC Microbiology
Eligibility Criteria
Education
Candidates must possess one of the following qualifications depending on role:
- B.Sc / M.Sc (Life Sciences / Microbiology / Biotechnology)
- B.Pharm / M.Pharm
- ITI (relevant trade)
- Diploma in Pharmacy or Engineering
- Any relevant science degree
Experience
- Freshers eligible for selected QC trainee roles
- 2–12 years of experience required for mid-level and senior roles
- Experience in sterile manufacturing (injectables, BFS, ophthalmics) preferred
Skills Required
- Knowledge of sterile manufacturing processes
- Understanding of cGMP and regulatory documentation
- Experience in equipment handling and validation (for relevant roles)
- Strong documentation and compliance skills
- Ability to work in regulated production environments
Note: As per company requirement, this hiring drive is open to male candidates only.
Salary & Benefits
- Salary: As per company norms and experience level
- Additional statutory and company benefits applicable
Freshers Jobs for Production QA QC Microbiology
Selection Process
The recruitment process includes:
- Direct walk-in interview
- Technical discussion
- HR interaction
Immediate joiners may receive preference.
How to Apply
Walk-In Interview Details
- Date: February 14, 2026 (Saturday)
- Time: 9:00 AM to 2:00 PM
- Venue: Plot No. 113-116, KIADB, 4th Phase, Bommasandra, Bangalore – 560099
Documents to Carry
- Updated resume
- Educational mark sheets and certificates
- Experience certificates (if applicable)
- Latest salary certificate
- Last three months’ payslips
Candidates unable to attend may send their resumes via email to the HR contact mentioned in the official notification. Freshers Jobs for Production QA QC Microbiology
Important Dates
- Walk-In Date: February 14, 2026
- Interview Time: 9:00 AM – 2:00 PM
- Positions will be filled based on immediate requirements
Why Apply for This Pharma Job?
- Opportunity to work with a reputed Indian pharmaceutical manufacturer
- Exposure to sterile injectables and ophthalmic manufacturing
- Openings for both freshers and experienced professionals
- Career growth in production and quality functions
- Stable work environment with regulatory-compliant systems
- Direct face-to-face interview process for faster selection
Freshers Jobs for Production QA QC Microbiology
Important Note / Disclaimer
IndiaPharmaJobs.in is an independent job information platform and is not affiliated with Micro Labs Limited. The above information is provided for awareness purposes only. Candidates are advised to verify details from official company sources before attending the walk-in. Freshers Jobs for Production QA QC Microbiology
Final Call-to-Action
If you are seeking opportunities in sterile pharmaceutical manufacturing in Bangalore, attend the walk-in interview with complete documentation and secure your chance to join Micro Labs Limited. Freshers Jobs for Production QA QC Microbiology


