Sun Pharma Hiring Executive Regulatory Affairs Job in Baroda for M.Pharm and M.Sc candidates with global submission experience and growth opportunities.
Sun Pharmaceutical Industries Ltd. is inviting applications for the position of Executive – Regulatory Affairs at its R&D facility in Baroda (Vadodara). This is an excellent opportunity for candidates with regulatory experience who want to work with one of India’s largest pharmaceutical companies. The role offers exposure to global regulatory submissions, lifecycle management, and dossier preparation for international markets.
If you are looking to grow your career in regulatory affairs within a reputed pharmaceutical organization, this opportunity can help you gain valuable global regulatory exposure. Sun Pharma Hiring Executive Regulatory Affairs Job
Company Overview
Sun Pharmaceutical Industries Ltd. is among the world’s leading specialty generics pharmaceutical companies with a strong global footprint. Headquartered in India, the company develops and manufactures a wide range of formulations and active pharmaceutical ingredients across multiple therapeutic segments.
Sun Pharma is widely recognized for its robust R&D infrastructure and global regulatory expertise. With a strong presence in regulated and emerging markets, the company offers excellent career opportunities for professionals seeking long-term growth in the pharmaceutical industry. Sun Pharma Hiring Executive Regulatory Affairs Job
Job Location & Employment Type
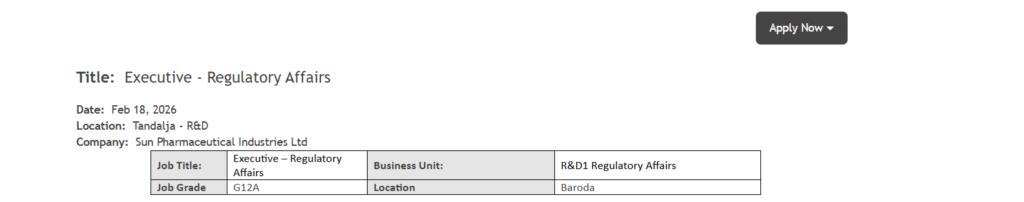
- Location: Baroda (Vadodara), Gujarat
- Work Location: Tandalja R&D Facility
- Employment Type: Full-Time
- Department: R&D Regulatory Affairs
- Job Grade: G12A
Open Position Details
- Job Title: Executive – Regulatory Affairs
- Business Unit: R&D Regulatory Affairs
- Market Focus: MENA / South Africa region
- Experience Level: 1–4 years
This role is ideal for professionals with hands-on experience in dossier preparation and international regulatory submissions. Sun Pharma Hiring Executive Regulatory Affairs Job
Key Roles & Responsibilities
The selected candidate will handle regulatory activities related to product registrations and lifecycle management. Core responsibilities include:
- Preparing and reviewing CMC (Chemistry, Manufacturing, and Controls) dossiers for new product submissions.
- Supporting regulatory filings for renewals and fresh product registrations.
- Reviewing technical documentation such as development reports, specifications, stability data, and scale-up reports.
- Evaluating artwork and packaging documents prior to exhibit batch initiation.
- Preparing responses to regulatory queries and deficiency letters.
- Managing product approvals by ensuring timely submission of clarifications and responses.
- Handling lifecycle management activities including post-approval variations.
- Preparing and reviewing variation filings based on country-specific regulatory requirements.
- Supporting changes related to API vendors, manufacturing sites, test parameters, or formulation updates.
- Maintaining regulatory documentation and approval records.
- Updating product information in central regulatory databases and repositories.
- Evaluating regulatory impact of change controls and variation proposals.
- Coordinating with internal stakeholders to ensure compliance and regulatory alignment.
This role requires strong documentation skills and familiarity with global regulatory guidelines. Sun Pharma Hiring Executive Regulatory Affairs Job
Eligibility Criteria
Education
Candidates must have one of the following qualifications:
- M.Pharm
- M.Sc (relevant life sciences discipline)
Experience
- 1–4 years of experience in Regulatory Affairs.
- Experience in international markets such as MENA, Africa, or emerging markets will be preferred.
Skills Required
- Knowledge of regulatory submission processes and dossier preparation.
- Strong understanding of CMC documentation.
- Familiarity with lifecycle management and variation filings.
- Awareness of global regulatory guidelines and compliance practices.
- Ability to review technical documents related to formulation and manufacturing.
- Strong written and verbal communication skills.
- Good coordination and stakeholder management abilities.
- Attention to detail in documentation and compliance tracking.
- Ability to work in cross-functional regulatory environments.
Sun Pharma Hiring Executive Regulatory Affairs Job
Salary & Benefits
- Salary: As per company norms
- Additional benefits may include structured career growth, learning opportunities, and exposure to global regulatory markets.
Selection Process
The recruitment process may include:
- Resume screening
- HR discussion
- Technical interview focusing on regulatory affairs knowledge
- Final selection
Candidates should be prepared to discuss regulatory submission formats, dossier modules, and lifecycle management concepts. Sun Pharma Hiring Executive Regulatory Affairs Job
How to Apply
Interested candidates should apply through the official Sun Pharma careers portal.
- Application Mode: Online Application
- Search for the role using the title “Executive – Regulatory Affairs” and apply through the official website.
Ensure your resume includes regulatory submission experience, markets handled, and dossier preparation exposure. Sun Pharma Hiring Executive Regulatory Affairs Job
Important Dates
- Candidates are advised to apply as early as possible.
Why Apply for This Pharma Job?
This role offers significant benefits for regulatory professionals aiming to grow in global pharma markets:
- Opportunity to work with India’s leading pharmaceutical company.
- Exposure to international regulatory markets and submissions.
- Hands-on experience in lifecycle management and variations.
- Strong learning environment within R&D regulatory teams.
- Career growth in global regulatory affairs.
- Valuable experience in dossier preparation and regulatory strategy.
If you want to build a long-term career in regulatory affairs with a reputed pharma leader, this role provides a strong platform.
Important Note
IndiaPharmaJobs.in is an independent job information platform created to help pharmaceutical and life sciences professionals discover genuine job opportunities. We are not affiliated with Sun Pharmaceutical Industries Ltd. Candidates are advised to apply only through official company channels and verify job details before applying. Sun Pharma Hiring Executive Regulatory Affairs Job
Final Words
Sun Pharmaceutical Industries Ltd. is offering an excellent opportunity for regulatory professionals to advance their careers in global submissions and lifecycle management. The Executive – Regulatory Affairs role at the Baroda R&D center provides exposure to international regulatory processes and hands-on involvement in dossier preparation. Candidates with relevant experience in regulatory documentation and submissions should apply online at the earliest to secure a role with one of the most respected pharmaceutical companies in the world. Sun Pharma Hiring Executive Regulatory Affairs Job


