Quality Assurance Executive Jobs at Zenotech Laboratories in Hyderabad for experienced pharma professionals with strong validation expertise and GMP compliance knowledge.
Company Overview
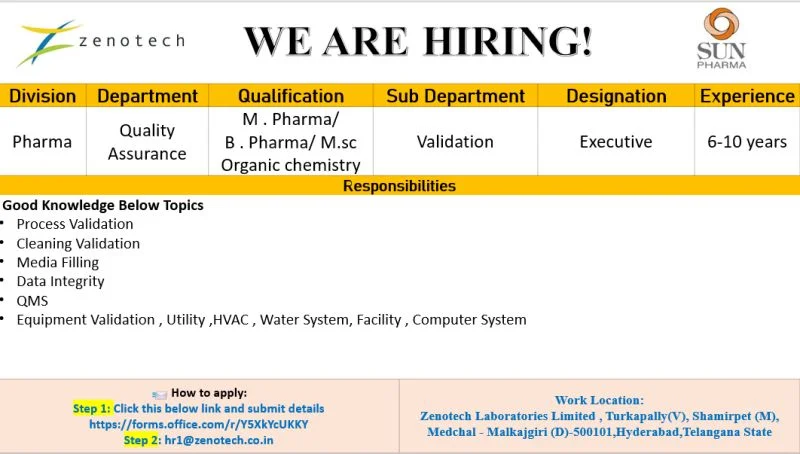
Zenotech Laboratories Limited is a well-established pharmaceutical manufacturing organization operating under the globally respected Sun Pharma Group. The company is recognized for its strong focus on quality-driven operations, particularly in the areas of injectables and biopharmaceutical products. With a firm commitment to regulatory compliance, innovation, and patient safety, Zenotech provides an excellent professional environment for experienced pharma professionals looking to advance their careers in quality assurance and validation functions. Quality Assurance Executive Jobs
Working at Zenotech means being part of a structured, GMP-compliant ecosystem where quality systems, continuous improvement, and regulatory excellence are core priorities. The organization offers exposure to advanced pharmaceutical technologies and stringent international quality standards. Quality Assurance Executive Jobs
Job Location & Employment Type
- Work Location: Turkapally Village, Shamirpet Mandal, Medchal-Malkajgiri District, Hyderabad, Telangana
- Employment Type: Full-Time
- Industry: Pharmaceutical Manufacturing (Injectables & Biopharmaceuticals)
Open Positions / Department Details
- Position Title: Executive – Quality Assurance (Validation)
- Department: Quality Assurance – Validation
- Number of Openings: Not Disclosed
- Reporting To: Senior QA / Validation Management
This position is ideal for experienced QA professionals who have hands-on exposure to validation activities in regulated pharmaceutical manufacturing environments. Quality Assurance Executive Jobs
Key Roles & Responsibilities
The selected candidate will play a critical role in maintaining and strengthening validation and quality systems at the manufacturing facility. Major responsibilities include, but are not limited to: Quality Assurance Executive Jobs
- Planning, executing, and documenting process validation activities for pharmaceutical manufacturing operations
- Preparing and reviewing validation protocols, reports, and related documentation as per GMP requirements
- Conducting cleaning validation studies to ensure equipment cleanliness and cross-contamination control
- Performing and evaluating media fill simulations to validate aseptic manufacturing processes
- Ensuring strict adherence to data integrity principles, including ALCOA+ guidelines
- Supporting the implementation and maintenance of Quality Management Systems (QMS)
- Executing equipment qualification and validation, including IQ, OQ, and PQ activities
- Handling validation of utilities and systems, such as HVAC, purified water systems, facilities, and computerized systems
- Participating in internal audits, regulatory inspections, and compliance-related activities
- Coordinating with cross-functional teams including production, engineering, and QC for validation execution
- Identifying gaps and recommending corrective and preventive actions (CAPA) for validation-related deviations
- Ensuring compliance with national and international regulatory guidelines applicable to pharmaceutical manufacturing
Eligibility Criteria
Education
- M.Pharm (Preferred)
- B.Pharm
- M.Sc (Organic Chemistry preferred)
Candidates must possess a strong academic background relevant to pharmaceutical quality and validation practices.
Experience
- Minimum 6 years and up to 10 years of experience in Quality Assurance with a strong focus on validation
- Prior experience in injectable manufacturing facilities or highly regulated environments is highly desirable
Skills Required
- In-depth knowledge of GMP, GDP, and regulatory compliance standards
- Strong understanding of validation lifecycle concepts
- Hands-on experience with process, cleaning, and equipment validation
- Knowledge of data integrity and documentation best practices
- Ability to prepare and review SOPs, protocols, and reports
- Good communication and coordination skills
- Strong analytical and problem-solving abilities
Salary & Benefits
- Salary: As per company norms
- Compensation will be aligned with industry standards and commensurate with experience and skill set
- Additional benefits may include health insurance, statutory benefits, performance-based incentives, and professional growth opportunities, as applicable under company policies
Quality Assurance Executive Jobs
Selection Process
- Initial resume shortlisting by the HR team
- Technical evaluation and interview with QA and validation leadership
- Final HR discussion
The selection process may vary depending on organizational requirements.
How to Apply
Interested and eligible candidates can apply through the following official channels:
- Online Application Form:
https://forms.office.com/r/yrXKkYcUKKY - Email Application:
Send your updated resume to hr1@zenotech.co.in
Candidates are advised to mention the position title clearly in the email subject line for faster processing. Quality Assurance Executive Jobs
Important Dates
- Last Date to Apply: Not Specified (Apply at the earliest for better consideration)
Why Apply for This Pharma Job?
This opportunity allows experienced QA professionals to work with a reputed Sun Pharma group company known for its regulatory excellence and quality culture. The role provides exposure to complex validation systems, injectables manufacturing, and stringent compliance environments. Professionals joining Zenotech can enhance their technical expertise, gain industry recognition, and build a stable long-term career in pharmaceutical quality assurance within a respected organization. Quality Assurance Executive Jobs
Important Note / Disclaimer
IndiaPharmaJobs.in is an independent pharmaceutical job information platform. We are not directly associated with Zenotech Laboratories Limited or the Sun Pharma Group. Job details are shared for informational purposes only, based on publicly available sources. Candidates are advised to verify all information directly with the employer before applying. Quality Assurance Executive Jobs
Final Call-to-Action
If you have strong experience in pharmaceutical validation and are looking to advance your career with a reputed organization in Hyderabad, apply now and take the next step in your professional journey. Quality Assurance Executive Jobs


