Work From Home Thermo Fisher Scientific Hiring Clinical Trial Coordination in India for clinical trial coordination, vendor oversight and eTMF management roles.
Company Overview
Thermo Fisher Scientific is a globally respected life sciences organization supporting pharmaceutical and biopharmaceutical companies in developing, managing, and delivering clinical trials across the world. Through its embedded Functional Service Provider (FSP) model, the company partners closely with leading biopharma organizations to strengthen study execution, operational efficiency, and regulatory compliance. Work From Home Thermo Fisher Scientific Hiring
In this role, professionals work as an extension of a global biopharmaceutical client’s study team, contributing directly to the successful delivery of clinical trials across multiple therapeutic areas and development phases. The organization emphasizes collaboration, accountability, innovation, and continuous professional growth. Work From Home Thermo Fisher Scientific Hiring
Job Location & Employment Type
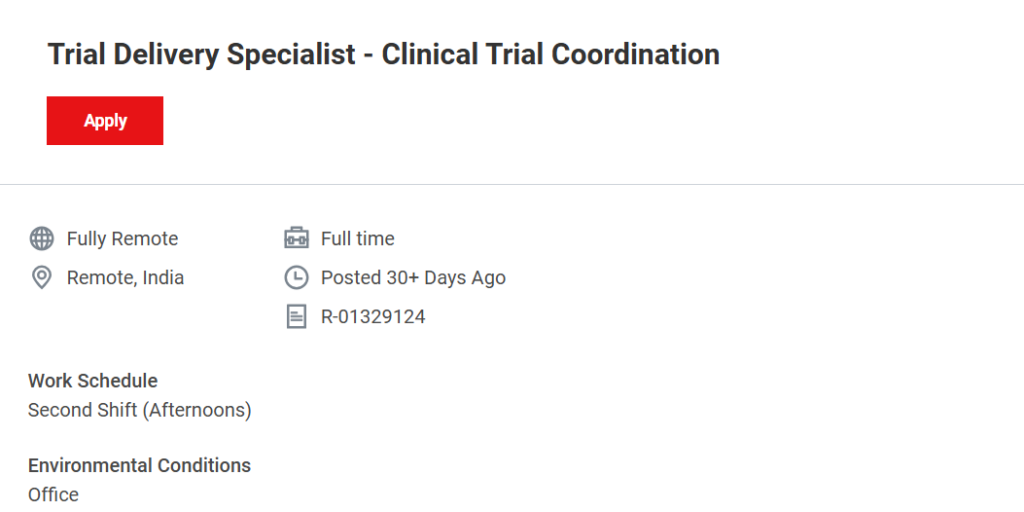
- Job Location: Remote – India
- Work Model: Fully Remote
- Employment Type: Full-time, permanent
- Work Schedule: Second Shift (Afternoon working hours)
- Functional Area: Clinical Trial Operations / Study Coordination
Open Positions / Department Details
Thermo Fisher Scientific is hiring for the role of Trial Delivery Specialist – Clinical Trial Coordination within its Global Study Delivery (FSP) team.
This position is designed for professionals with strong clinical trial coordination and operational oversight experience who can support global studies by ensuring smooth execution, documentation integrity, and cross-functional collaboration throughout the trial lifecycle. Work From Home Thermo Fisher Scientific Hiring
Key Roles & Responsibilities
The Trial Delivery Specialist plays a critical execution-focused role in ensuring high-quality clinical study delivery across all phases of development. Work From Home Thermo Fisher Scientific Hiring
Primary Responsibilities Include:
- Partnering closely with Global Study Leaders to track study progress, milestones, and operational performance
- Identifying potential risks related to quality, timelines, or budgets and escalating issues with proposed mitigation strategies
- Supporting end-to-end operational study activities from study start-up through close-out and archival
- Setting up, maintaining, and ensuring accuracy of study systems, trackers, internal databases, and project plans
- Reviewing essential clinical documents such as study protocols and informed consent forms
- Supporting the development and maintenance of operational plans, including monitoring strategies, vendor management plans, deviation management plans, and risk management plans
- Coordinating cross-functional study team activities, including meeting preparation, scheduling, documentation, and follow-up actions
- Acting as a communication bridge between internal teams and external partners such as CROs, regulatory teams, local country operations, and third-party vendors
- Supporting country-level oversight by tracking recruitment metrics, data completeness, protocol deviations, compliance status, local budgets, and regulatory documentation
- Serving as a primary point of contact for assigned countries and functional stakeholders
Vendor Oversight & Clinical Supplies Management
Vendor and CRO Oversight:
- Managing relationships with CROs and external service providers
- Monitoring vendor performance and ensuring timely delivery of study-specific milestones
- Reviewing deliverables and addressing quality or timeline issues proactively
Clinical Supplies Oversight:
- Coordinating the delivery of investigational products (IP), clinical supplies, and study materials
- Monitoring supply continuity and escalating risks that may impact trial conduct
- Supporting resolution of supply-related challenges in collaboration with study teams
eTMF & Data Oversight Responsibilities
- Ensuring electronic Trial Master File (eTMF) completeness and inspection readiness
- Supporting eTMF setup, periodic reviews, and quality checks
- Following up on missing or incomplete documentation with internal and external stakeholders
- Supporting safety report dissemination and document verification activities
- Maintaining high standards of data quality and regulatory compliance
Work From Home Thermo Fisher Scientific Hiring
Budget & Financial Coordination
- Supporting study financial activities, including change orders and expense tracking
- Ensuring alignment between contracts, budgets, and internal systems
- Escalating financial inconsistencies or risks to study stakeholders in a timely manner
Eligibility Criteria
Education
- Bachelor’s degree in Life Sciences, Pharmacy, Clinical Research, or related disciplines
- Advanced degrees or clinical research certifications will be an added advantage
Experience
- Prior experience in clinical trial coordination, study operations, or global study support roles
- Hands-on exposure to global clinical trials across multiple phases
- Experience working with CROs, vendors, and cross-functional clinical teams
- Familiarity with FSP or sponsor-embedded models is preferred
Work From Home Thermo Fisher Scientific Hiring
Skills Required
- Strong understanding of clinical trial operations and study lifecycle management
- Knowledge of ICH-GCP guidelines, regulatory documentation, and inspection readiness
- Experience with eTMF systems and clinical trial databases
- Excellent organizational, planning, and multitasking abilities
- Strong written and verbal communication skills
- Ability to work independently in a remote, global team environment
- Analytical mindset with strong risk identification and problem-solving skills
- Comfort working across different time zones and cultures
Work From Home Thermo Fisher Scientific Hiring
Salary & Benefits
Salary: As per company norms and aligned with experience and role responsibilities
Benefits May Include (as per company policy):
- Competitive compensation structure
- Fully remote work opportunity
- Exposure to global biopharmaceutical studies
- Structured learning, mentorship, and career development programs
- Opportunities for internal career progression across clinical operations roles
Selection Process
The selection process generally involves:
- Profile shortlisting based on clinical operations experience
- Technical and functional interviews with global study delivery teams
- Final HR discussion and role confirmation
Only shortlisted candidates will be contacted for further stages.
How to Apply
Interested candidates must apply online only through the official careers portal.
- Job Requisition ID: R-01329124
- Mode of Application: Online application
Ensure your resume clearly reflects your experience in clinical trial coordination, vendor oversight, and global study operations. Work From Home Thermo Fisher Scientific Hiring
Important Dates
- Last Date to Apply: Not specified
Early applications are encouraged due to ongoing shortlisting.
Why Apply for This Pharma Job?
This role offers a unique opportunity to work remotely while contributing to global clinical trials that support life-changing therapies. The Trial Delivery Specialist position provides end-to-end exposure to study operations, collaboration with international stakeholders, and involvement in cutting-edge clinical trial technologies. With multiple future career pathways available in project management, study operations, or clinical research roles, this position is ideal for professionals seeking long-term growth in global clinical research. Work From Home Thermo Fisher Scientific Hiring
Important Note / Disclaimer
IndiaPharmaJobs.in is an independent job information platform and is not affiliated with Thermo Fisher Scientific, its clients, or recruitment partners. All job details are shared for informational purposes only. Candidates are advised to verify information independently and should never pay any fees for job applications or recruitment processes. Work From Home Thermo Fisher Scientific Hiring
Final Call-to-Action
Experienced clinical trial professionals looking for remote global study coordination roles are encouraged to apply without delay. Work From Home Thermo Fisher Scientific Hiring


